The Epidiolex Effect: Will Other CBD Drugs Receive FDA and DEA Approval?
Though it doesn’t get users high like THC-dominant products, CBD is all the rage these days due to its medical benefits and gray-area legality.
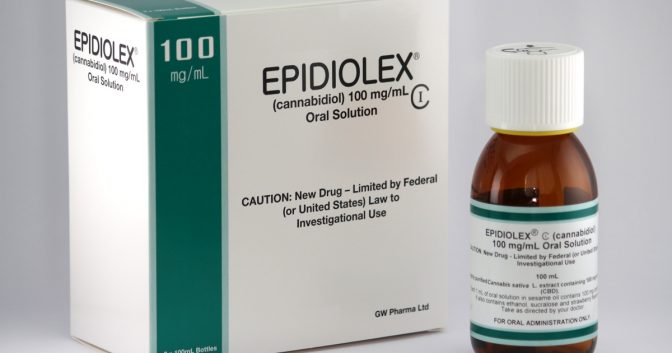
On September 28, the DEA designated Epidiolex—a plant-based CBD pharmaceutical manufactured by the UK-based GW Pharmaceuticals—a Schedule V drug in the government’s list of controlled substances. All other CBD formulations remain in Schedule I, which is for drugs with a “high potential for abuse” and “no currently accepted medical use.” Schedules III to V are for “drugs with lower potential for abuse.”
This move reinforced a federal ruling on April 16 that affirmed the December 2016 DEA decision to create a new Schedule I code for cannabis extracts, including CBD extracts. The FDA’s Peripheral and Central Nervous System Drugs Advisory Committee concluded that Epidiolex was safe, effective and had negligible abuse potential. On the basis of their review, the agency approved Epidiolex for use in treating rare forms of childhood epilepsy (Lennox-Gastaut syndrome and Dravet syndrome).
A month later, on May 16, the Department of Health and Human Services approved the FDA’s Epidiolex recommendation.
Frontier Data predicts total sales of all CBD products in the U.S. will top $1.9 billion by 2022.
On July 23, the World Health Organization’s Expert Committee on Drug Dependence stated that “no public health problems have been associated with CBD use” and that “CBD has been found to be generally well tolerated with a good safety profile.” Tasked with deciding whether CBD should be added to the international schedule of controlled substances, the committee recommended that “preparations considered to be pure CBD should not be scheduled.”
The U.S. schedules were created by the Controlled Substances Act (CSA) in 1970 and are interpreted and enforced by the DEA. CBD is not specifically listed by the CSA, though it falls under the definition of “marijuana extract” and thus is effectively in Schedule I.
All this legal wrangling ignores a reality on the ground: CBD products are offered for sale at dispensaries, regular shops and via mail order in most parts of the United States. However, CBD products made from cannabis grown for medical or adult social use have to be sold through licensed dispensaries or adult-use stores in legal states. CBD products derived from industrial hemp are available in most states either over the counter or online.
While GW Pharmaceuticals’ Epidiolex has received a Schedule V designation from the DEA, all other CBD formulations remain in Schedule I.
“The fact of the matter is CBD has broken through, even though it’s still federally illegal,” Martin A. Lee, co-founder and executive director of Project CBD, told the Century of Lies podcast on August 28. “Yet it’s everywhere, at the community market, at Whole Foods, at gas stations, at massage studios. It’s illegal and it’s everywhere.”
The push for CBD is not surprising, whether it’s sold as a pharmaceutical drug or as a “dietary supplement” similar to gingko biloba or St. John’s wort. Frontier Data predicts total sales of all CBD products in the U.S. will top $1.9 billion by 2022. Sales of the pharmaceutical CBD product Epidiolex are expected to comprise less than a third of that projected total.
Frontier’s estimate is actually fairly conservative. Epidiolex may only currently be approved by the FDA for treating childhood epilepsy, but there are many other potential uses for CBD medicines, such as in the treatment of substance abuse disorders including opioid use disorders.
PROJECT CBD’S MARTIN A. LEE: “The fact of the matter is CBD has broken through. It’s illegal and it’s everywhere.”
In its September 2018 issue, Frontiers in Immunology published an analysis of CBD research that found “CBD was shown to have anxiolytic, antipsychotic and neuroprotective properties. In addition, basic and clinical investigations on the effects of CBD have been carried out in the context of many other health conditions, including its potential use in epilepsy, substance abuse and dependence, schizophrenia, social phobia, post-traumatic stress, depression, bipolar disorder, sleep disorders and Parkinson’s.”
Ultimately, state regulations, not the Feds, may force the issue regarding CBD products. The California Department of Health issued a FAQ in July that clearly and repeatedly stated industrial hemp-derived CBD products are not an approved food, food ingredient, food additive or dietary supplement. Legally, all CBD products in California must come through the regulated adult-use or medical programs. What other states will do is an open question; however, since states derive tax revenue from those regulated programs, the chances are good that where California leads, the rest of the states will follow.
More CBD Coverage
CBD Helps Musician Recover from Brain Cancer
FDA Warns Four CBD Producers About Making Medical Claims
Target Pulls CBD Products After Offering Them Online
Dr. Frank D’ambrosio: The ABCs of CBD
If you enjoyed this Freedom Leaf article, subscribe to the magazine today!

